Fine Beautiful Info About How To Increase Buffer Capacity

It depends on the amount of acid and base used to prepare the buffer.
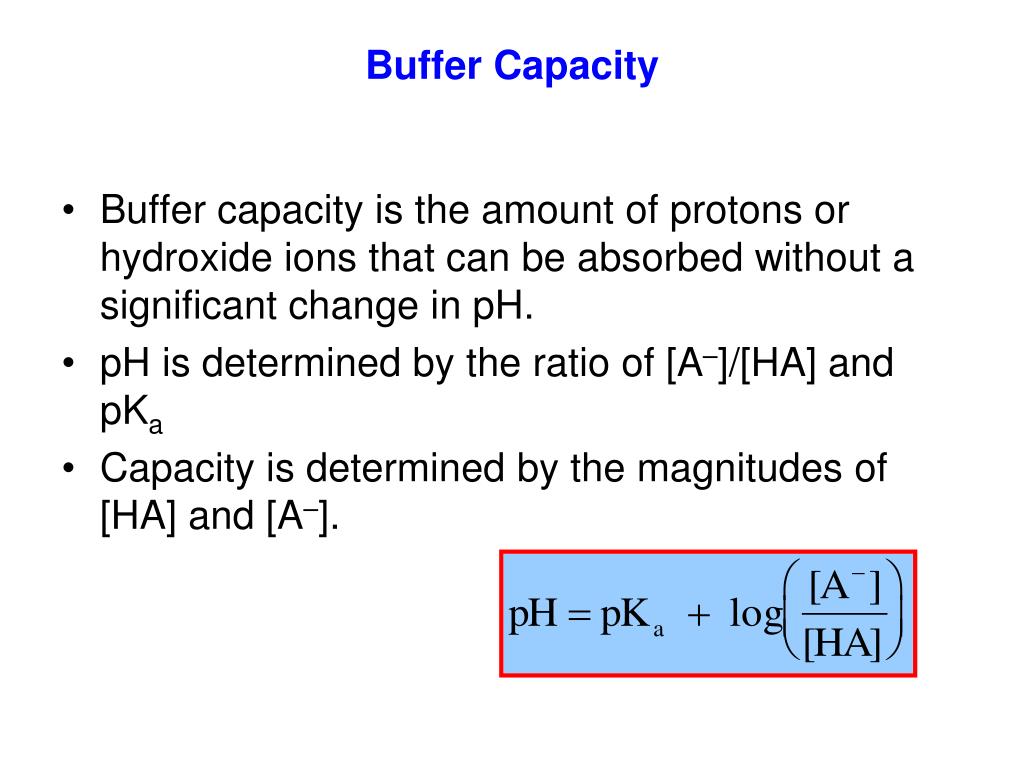
How to increase buffer capacity. $$β=\frac{δ(\ce{h+})}{δ(\mathrm{ph})}$$ specifically the amount of acid/base that needs. Buffer capacity is the amount of a strong acid or base a buffer can neutralize before a significant change in its ph. The capacity of a buffer solution to maintain a constant ph depends on the specific components of the buffer, it’s concentration and the amounts of acid or base challenging.
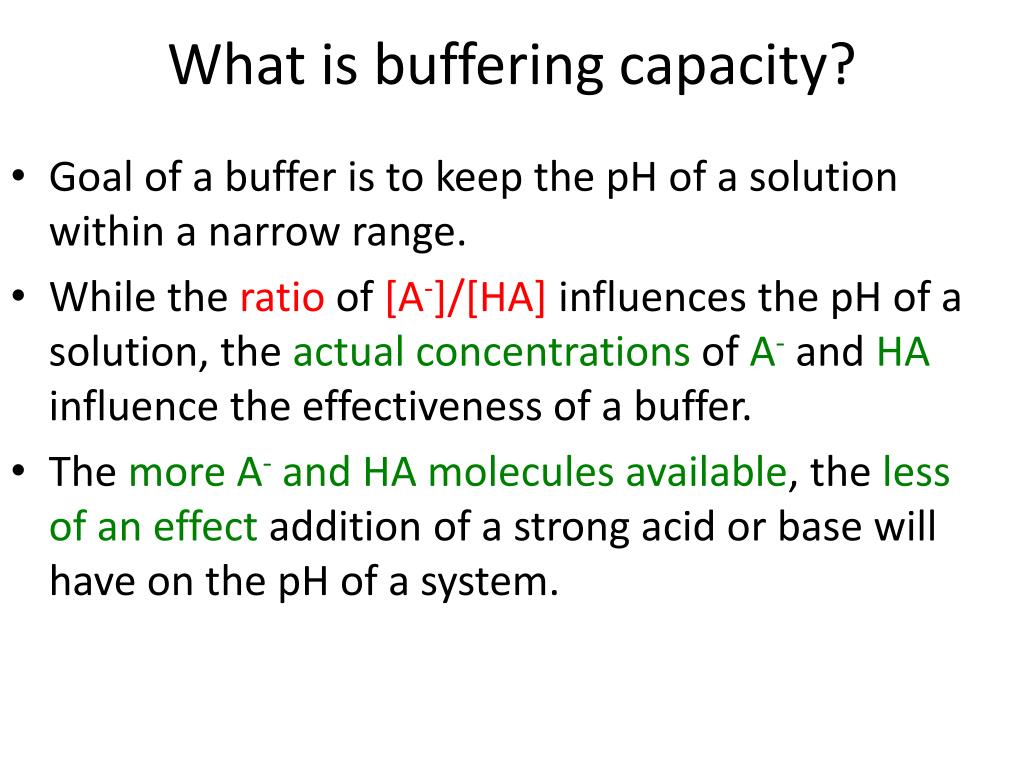
When [ha] is greater than [a⁻], the capacity is higher for added base than. My idea is to add some grams of. Every buffer that is made has a certain buffer capacity, and buffer range.
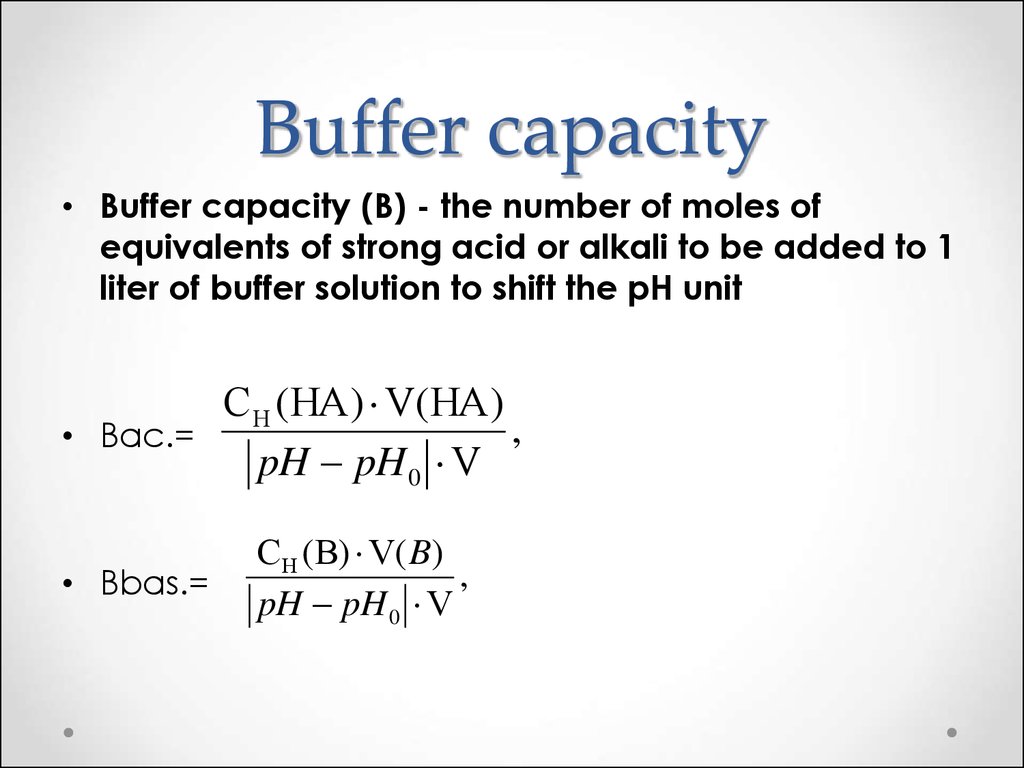
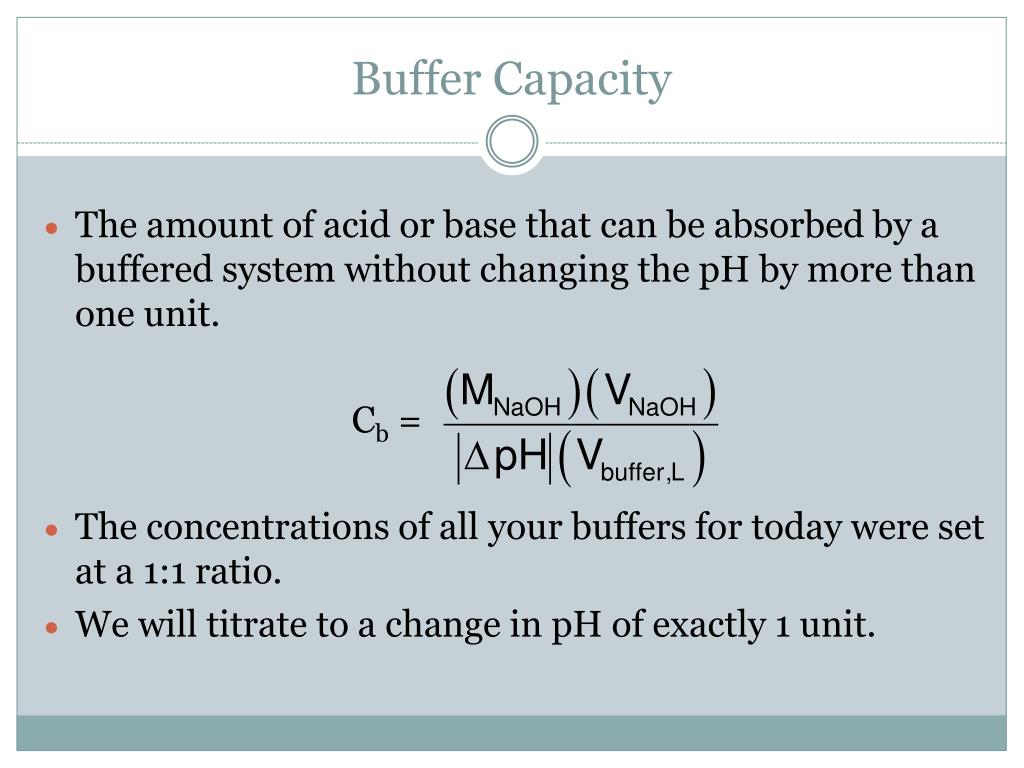
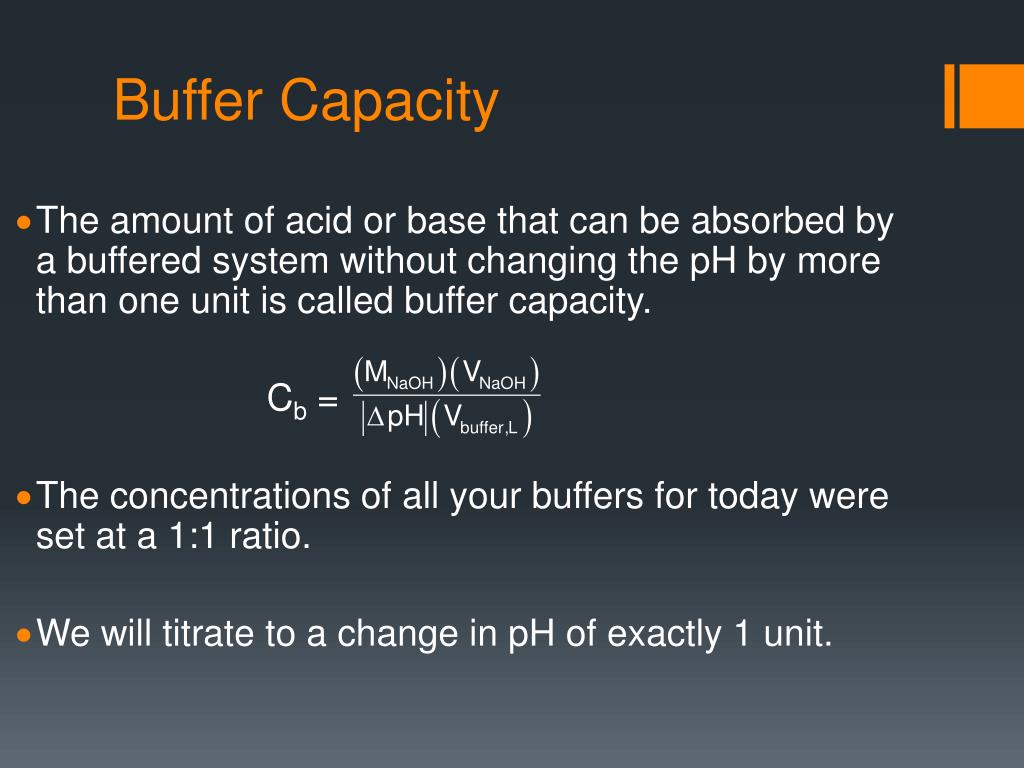
This turns out to be the case when the concentrations of the conjugate acid and conjugate base are approximately equal (within about a factor of 10). Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it will. Buffer capacity (β) is defined as the moles of an acid or base necessary to change the ph of a solution by 1, divided by the ph change and the volume of buffer in liters;
The more concentrated the buffer mixture, the higher the buffer capacity. Therefore, the buffer capacity increases both with higher. I know buffer capacity is the following:
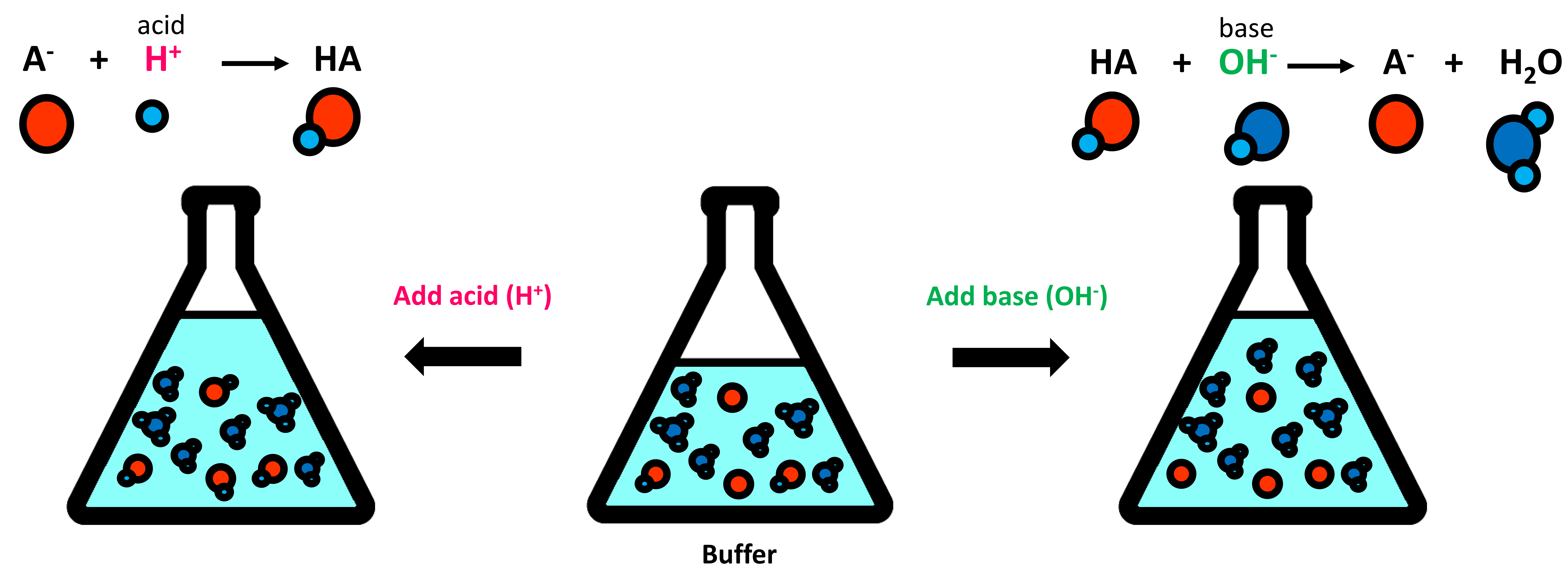
A solution with more weak acid, [ha], has a higher buffer capacity for addition of strong base. Buffer capacity depends on the concentrations of the species in the solution; Buffer capacity is a property of a buffer and it tells you how much acid or base you can add before the ph starts changing.
For a given ratio of [ha] to [a⁻], the greater the concentrations, the higher the overall buffer capacity. Buffers function best when the pka of the conjugate weak acid used is close to the desired working range of the buffer. Generally, the addition of strong acids or bases to a solution changes the ph dramatically because the acid or base reacts with the water molecules in solution, increasing the.
Taking buffering capacity to mean the amount of acid or base required to change $\mathrm{ph}$ by a fixed small amount at $\mathrm{ph}$ values near one of. So, in order to be an effective buffer, the. What kind of solution would act as a buffer solution and why, how to increase buffer capacity, how to c.
Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or. A buffer is a special solution that stops massive changes in ph levels. Basically, as your buffer capacity goes up, which i'm.
Buffer capacity is the most straightforward way to enhance resilience, whether in the form of underutilized. For example, we know the k a for. A buffer solution is a solution that only changes slightly when an acid or a base is added to it.
How do i increase buffer size on my pc? How does a buffer maintain ph?